Abstract
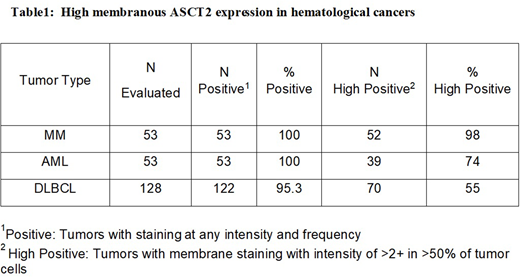
The neutral amino acid transporter, ASCT2, is frequently overexpressed in several cancers to sustain "glutamine addiction" of cancer cells. High expression of ASCT2 is often associated with poor disease prognosis. Immuno-histochemistry (IHC) on formalin-fixed paraffin embedded (FFPE) tissue samples revealed high prevalence of membranous ASCT2 expression in several hematological cancers, including MM, AML, and DLBCL summarized in Table1. ASCT2 expression was predominantly on the plasma membrane of the carcinoma cells. ASCT2 staining was observed in almost all the samples with high positivity (>2+ and in >50% of tumor cells) in 98, 74 and 55% of MM, AML and DLBCL samples respectively. Additionally, flow cytometry analyses suggest significantly high expression of ASCT2 in bone marrow (BM) samples from AML and MM patients in comparison to BM from healthy donors. Also, our data suggest relatively less expression of ASCT2 in LT-HSC (long term hematopoietic stem cells) in comparison to prominent myeloid-associated marker CD33. In contrast, we found similar expression profile of ASCT2 and CD33 in downstream lineage-committed progenitor cells, such as MPP (multi potent progenitors) and CP (common progenitors). Furthermore, IHC evaluated across normal tissues suggest restricted ASCT2 expression in the normal tissues of vital organs.
Broad expression across various unmet cancers and restricted expression in normal tissues warrant ASCT2 as an attractive candidate for an antibody drug conjugate. MEDI7247 is a novel investigational antibody-drug conjugate (ADC) comprising an anti-ASCT2 human monoclonal antibody site-specifically conjugated to pyrrolobenzodiazepine (PBD) dimer via a protease-cleavable linker, with a drug to antibody ratio (DAR) of 2. MEDI7247 specifically binds to the cell surface ASCT2 while exhibiting no affinity to the other members of the family, including ASCT1. Following binding to the extracellular region of ASCT2 antigen, MEDI7247 is internalized and trafficked to the lysosomes to subsequently release the PBD warhead. The release of PBD induces DNA damage and results in tumor cell death.
MEDI7247 shows potent in vitro cytotoxic killing in several heme cancer cell lines (IC50 range of 0.05 ng/ml to ~65 ng/ml) with variable levels of ASCT2 expression (e.g. H929 (high) ~ 1.1 X106 copies/cell; Nomo (low) ~ 1.3 X105 copies/cell). In vivo anti-tumor activity of MEDI7247 was evaluated in a panel of hematological tumor models (CDx) representing AML, MM, DLBCL, cALL, and Burkitt's lymphoma. Briefly, MEDI7247 (dosed: single dose or Q1Wx4) demonstrated a significant survival advantage in disseminated or subcutaneous tumor models, when compared to the untreated control or standard of care (SOC) at the various dose levels examined: 0.05, 0.1 and 0.4 mg/kg, respectively. Likewise, MEDI7247 was also tested in disseminated AML PDX (ASCT2-low) models. Significant improvement in survival was observed at both 0.1 and 0.4 mg/kg with the higher dose level extending survival by >80 days and PDX models showed robust antitumor effect and significant survival benefit compared to standard of care (SOC).
We used flow cytometry sorting of CD34+ and CD38+ populations from AML BM and ran colony forming assays in methocult culture conditions to confirm that CD34+CD38+ cells are leukemic stem cells (LSC) of AML BM samples. Furthermore, we confirmed high expression of ASCT2 in LSC and in the bulk population of AML BM. Similar analyses suggest relatively high expression of ASCT2 in MM plasma cells (CD138+CD19-) and modest to low expression in MM stem cells (CD19+ CD138-). This may yield opportunity for more durable clinical response in AML, genetically heterogeneous diseases. Furthermore, MEDI7247 demonstrated acceptable safety profile in toxicity studies with non-human primates to support first in human trials.
In conclusion, based on its combined efficacy and safety, MEDI7247, a first-in class ADC is currently being evaluated in clinic for the treatment of ASCT2 positive hematological malignancies (NCT03106428).
Pore:Medimmune: Employment. Schifferli:Medimmune: Employment. Monks:Medimmune: Employment. Tammali:Medimmune: Employment. Borrok:Medimmune: Employment. Hurt:Medimmune: Employment. Flynn:Medimmune: Employment. Rebelatto:Medimmune: Employment. Townsley:Medimmue: Employment. Hinrichs:Medimmune: Employment. Dixit:Medimmune: Employment. Coats:Medimmune: Employment. Herbst:Medimmune: Employment. Tice:Medimmune: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal